您好,欢迎来到显微镜配件耗材官网!
 最新资讯返回
最新资讯返回
双光子显微镜由于其对厚的离体组织以及直接能进行在体动物细胞水平高分辨率观察而深受神经科学,胚胎学,肿瘤学和免疫学家的青睐。目前已经成为活体状态下研究深层组织高分辨率形态结构和生理变化的核心工具。然而,深层组织的散射极大地限制了双光子显微镜在鼠脑脑皮层的穿透深度。目前采集细胞级分辨率水平的脑皮层下图像(如海马区)需要移除上层脑组织或者插入光学探头才能实现。
第一篇 三光子观察小鼠脑白质以及海马区神经元和皮层下血管
光学图像以其无损或最小损伤的显微图像呈现生物组织而在生物研究和临床诊断方面占据极为重要的地位。但由于生物组织的异质性和强烈的散射极大地影响了光学成像的深度,因此高分辨率光学成像一直限制在薄的组织片或表面组织。虽然双光子的出现大大扩展了高分辨率光学图像的穿透深度,尤其是活体应用。在从双光子发明的20多年里,它已经在许多领域第一次在自然环境下观察细胞的正常行为和对外界条件刺激的反应。但双光子激发荧光分子依赖足够的激发光强度到达物镜无散射条件下的焦点位置,高分辨双光子图像图像深度限制主要由在散射生物组织中的激发的信号背景比决定。也就是双光子大约能从组织表面穿透5到6 倍的有效衰减长度(le)那么深。举例说,对于775nm激发小鼠脑新皮层(le ∼130 μm)的双光子图像,深度限制大约在700um。
要想在体条件下看到更深层的图像,一个有效的方法就是使用更长波长的激发光降低激发光在组织中的被散射衰减程度。最佳的激发光波长窗口是由组织对光散射(波长越长,散射越弱)和水对光的吸收(尤其对1100-2000nm波长光有较强的吸收)的平衡来决定的。因此考虑到吸收和散射的综合因素,1300 nm和1700nm附近波长是两个最佳激发窗口。(如图1)
根据光学理论计算,1700nm如果采用双光子激发,相当于850nm的单光子激发;采用三光子激发,相当于566nm的单光子激发。因此从荧光检测方便性,荧光染料或荧光蛋白的选择以及信号背景比等角度看,相比双光子,1700nm的三光子激发是最好的解决方案。
虽然三光子从上世纪90年代后就陆续有应用(主要使用690-900nm激发),但三光子一个非常明显的优势确经常被忽略,那就是三光子的激发强度在远离激发焦面位置是呈四次方衰减,而双光子是呈平方衰减。换句话说,就是三光子激发相比双光子激发,能明显降低非焦区域的背景噪声,极大地提高信号背景比。
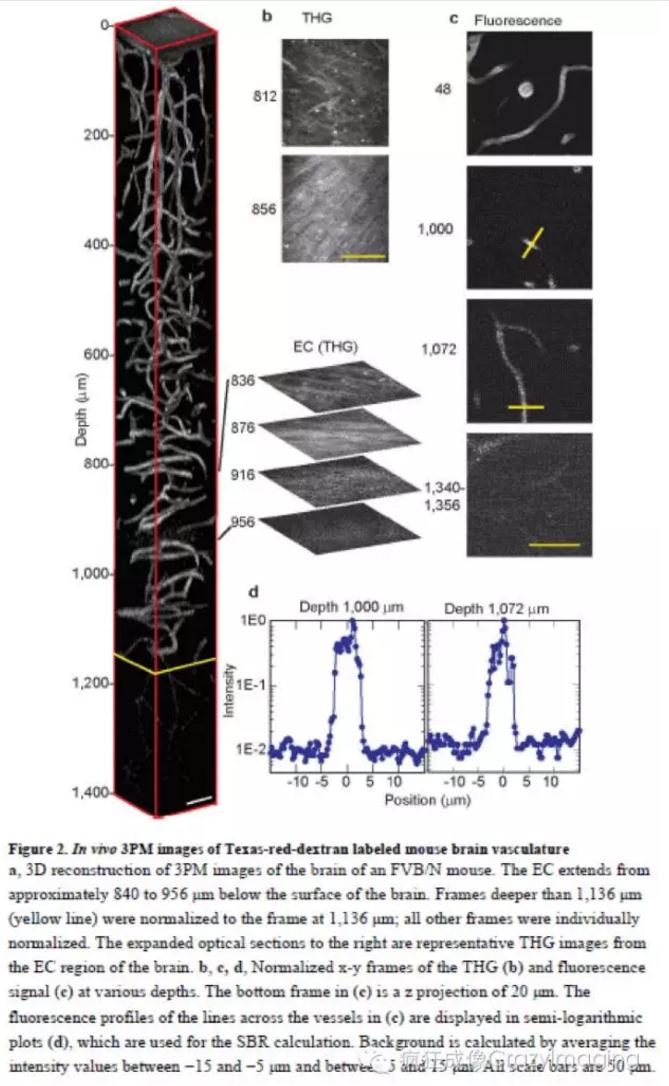
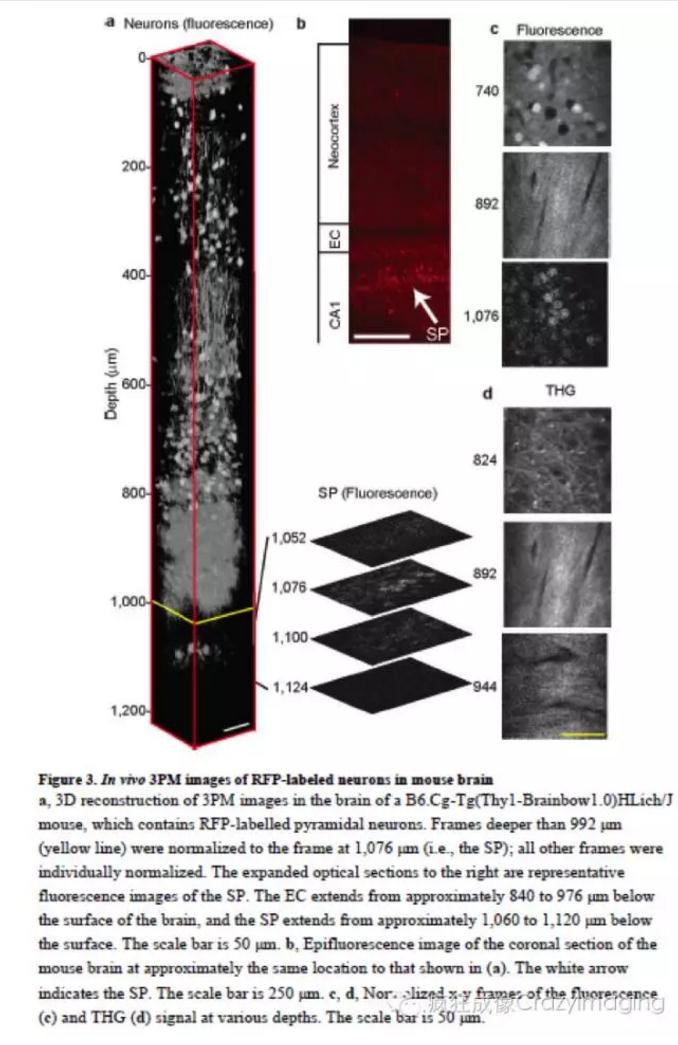
因此,我们使用自己研发的1,675 nm全新高脉冲能量激光(但平均功率仍保持较低水平,1MHz频率下输出能量67nJ,脉宽65fs,脉宽压缩比6倍)进行三光子激发,活体条件下观察采集到鼠脑1400um厚度的Z轴序列图像(4um步进)并进行了数据的3D重构。从图像中可清晰地看到活体小鼠脑神经元白质层中的轴突外囊(从皮层表面下840um开始,116um厚,三次谐波),海马的CA1 区神经元(从皮层表面下956um,RFP),以及深达皮层下1300um的血管(dextran-coupledTexas Red dye)的高分辨,高反差图像。显示了三光子与双光子成像相比,尤其是采集深度和信号背景比上更为强大的能力。(如图2和图3)
References
1. Denk W, Strickler JH, Webb WW. Two-photon laserscanning fluorescence microscopy. Science. 1990; 248:73–76. [PubMed: 2321027]
2. Kerr JND, Denk W. Imaging in vivo: watching the brainin action. Nat Rev Neurosci. 2008; 9:195–205. [PubMed: 18270513]
3. Dombeck DA, Harvey CD, Tian L, Looger LL, Tank DW.Functional imaging of hippocampal place cells at cellular resolution duringvirtual navigation. Nat Neurosci. 2010; 13:1433–1440. [PubMed: 20890294]
4. Olivier N, et al. Cell lineage reconstruction of earlyzebrafish embryos using label-free nonlinear microscopy. Science. 2010;329:967–971. [PubMed: 20724640]
5. Williams RM, et al. Strategies for high-resolutionimaging of epithelial ovarian cancer by laparascopic nonlinear microscopy.Transl Oncol. 2010; 3:181–194. [PubMed: 20563260]
6. Jung JC, Mehta AD, Aksay E, Stepnoski R, Schnitzer MJ.In vivo mammalian brain imaging using one- and two-photon fluorescencemicroendoscopy. J Neurophysiol. 2004; 92:3121–3133. [PubMed: 15128753]
7. Levene MJ, Dombeck DA, Kasischke KA, Molloy RP, WebbWW. In vivo multiphoton microscopy of deep brain tissue. J Neurophysiol. 2004;91:1908–1912. [PubMed: 14668300]
8. Kleinfeld D, Mitra PP, Helmchen F, Denk W.Fluctuations and stimulus induced changes in blood flow observed in individualcapillaries in layers 2 through 4 of rat neocortex. Proc Natl Acad Sci U S A.1998; 95:15741–15746. [PubMed: 9861040]
9. Svoboda K, Helmchen F, Denk W, Tank DW. Spread ofdendritic excitation in layer 2/3 pyramidal neurons in rat barrel cortex invivo. Nat Neurosci. 1999; 2:65–73. [PubMed: 10195182]
10. Theer P, Hasan MT, Denk W. Two-photon imaging to adepth of 1000 μm in living brains by use of a Ti:Al2O3 regenerative amplifier. Opt Lett. 2003;28:1022–1024. [PubMed: 12836766]
11. Kobat D, et al. Deep tissue multiphoton microscopyusing longer wavelength excitation. Opt Express. 2009; 17:13354–13364. [PubMed:19654740]
12. Kobat D, Horton NG, Xu C. In vivo two-photonmicroscopy to 1.6-mm depth in mouse cortex. J Biomed Opt. 2011; 16:106014.[PubMed: 22029361]
13. Helmchen F, Denk W. Deep tissue two-photonmicroscopy. Nat Methods. 2005; 2:932–940. [PubMed: 16299478]
14. Balu M, et al. Effect of excitation wavelength onpenetration depth in nonlinear optical microscopy of turbid media. J BiomedOpt. 2009; 14:010508. [PubMed: 19256688]
15. Sacks ZS, Kurtz R, Juhasz T, Spooner G, Mouroua GA.Subsurface Photodisruption in Human Sclera: Wavelength Dependence. OphthalmicSurg Lasers Imaging. 2003; 34:104–113. [PubMed: 12665225]
16. Kou L, Labrie D, Chylek P. Refractive indices ofwater and ice in the 0.65- to 2.5-μm spectral range. Appl Opt. 1993;32:3531–3540. [PubMed: 20829977]
17. Xu C, Zipfel W, Shear JB, Williams RM, Webb WW.Multiphoton fluorescence excitation: new spectral windows for biologicalnonlinear microscopy. Proc Natl Acad Sci U S A. 1996; 93:10763–10768. [PubMed:8855254]
18. Hell SW, et al. Three-photon excitation influorescence microscopy. J Biomed Opt. 1996; 1:71–74. [PubMed: 23014645]
19. Wokosin DL, Centonze VE, Crittenden S, White J.Three-photon excitation fluorescence imaging of biological specimens using anall-solid-state laser. Bioimaging. 1996; 4:208–214.
20. Zysset B, Beaud P, Hodel W. Generation of opticalsolitons in the wavelength region 1.37–1.49 μm. Appl Phys Lett. 1987;50:1027–1029.
21. Wang K, Xu C. Tunable high-energy soliton pulsegeneration from a large-mode- area fiber and its application to third harmonicgeneration microscopy. Appl Phys Lett. 2011; 99:071112.
22. Limpert J, et al. High-power rod-type photoniccrystal fiber laser. Opt Express. 2005; 13:1055–1058. [PubMed: 19494970]
23. Barad Y, Eisenberg H, Horowitz M, Silberberg Y.Nonlinear scanning laser microscopy by third harmonic generation. Appl PhysLett. 1997; 70:922–924.
24. Müller M, Squier J, Wilson KR, Brakenhoff GJ. 3Dmicroscopy of transparent objects using third-harmonic generation. J Microsc.1998; 191:266–274. [PubMed: 9767491]
25. Farrar MJ, Wise FW, Fetcho JR, Schaffer CB. In vivoimaging of myelin in the vertebrate central nervous system using third harmonicgeneration microscopy. Biophys J. 2011; 100:1362–1371. [PubMed: 21354410]
26. Livet J, et al. Transgenic strategies forcombinatorial expression of fluorescent proteins in the nervous system. Nature.2007; 450:56–62. [PubMed: 17972876]
27. Franklin, KBJ.; Paxinos, G. Mouse brain instereotaxic coordinates. Academic Press; London: 2008.
28. Murphy PA, et al. Notch4 normalization reduces bloodvessel size in arteriovenous malformations. Sci Transl Med. 2012; 4:117ra8.
29. Pologruto TA, Sabatini BL, Svoboda K. ScanImage:flexible software for operating laser scanning microscopes. Biomed Eng Online.2003; 2:13. [PubMed: 12801419]
30. Binding J, et al. Brain refractive index measured invivo with high-NA defocus corrected full-field OCT and consequences fortwo-photon microscopy. Opt Express. 2011; 19:4833–4847. [PubMed: 21445119]
31. Bacallao, R.; Sohrab, S.; Phillips, C. GuidingPrinciples of Specimen Preservation for Confocal Fluorescence Microscopy. In:Pawley, JB., editor. Handbook Of Biological Confocal Microscopy. p. 368-380.
32. Rosidi NL, et al. Cortical microhemorrhages causelocal inflammation but do not trigger widespread dendrite degeneration. PlosOne. 2011; 6:e26612. [PubMed: 22028924]